"But that sample passed... why can't we just use that result?"
The room went silent.
This wasn't a question from a junior analyst. It came from a senior executive.
I was stunned.
It highlighted a concerning mindset that still lingers in the C-suite: The idea that Quality Control is about "hunting for a pass" rather than establishing the truth.
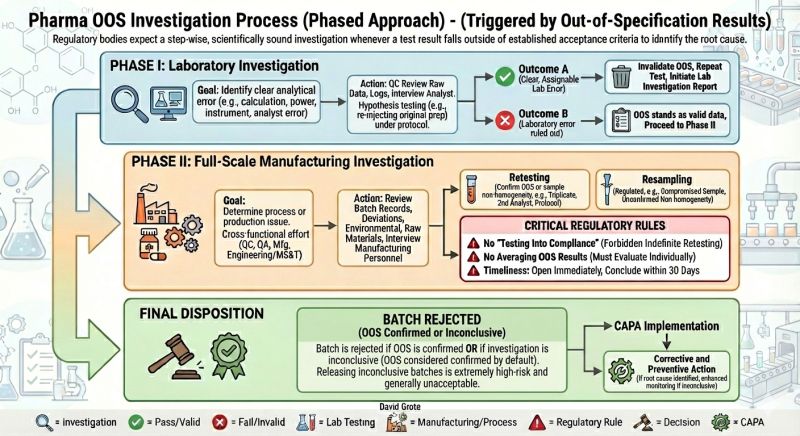
In a GxP environment, an OOS is not just a data point to be managed; it is a potential patient safety risk.
When leadership pushes to "test into compliance," they aren't just breaking laws; they are putting patients at risk, and are inviting a Warning Letter.
Regulatory bodies (FDA, EMA) demand a scientifically sound, phased approach; not a cherry-picked one. Our patients deserve nothing less.
Here is the reality we need to reinforce:
Inconclusive = Confirmed. If you cannot prove a lab error (Phase I) or a process error (Phase II), the failure stands. You cannot release a batch based on hope.
Retesting is not Resampling. Retesting confirms a result; resampling is strictly regulated and rarely permitted. Knowing the difference saves you from consent decrees.
No Averaging. You cannot 'dilute a failure' by averaging it with passing retests.
If the 'top floor' doesn't respect the investigation process, the 'shop floor' won't either.
The goal is never just "a passing result." The goal is Root Cause identification and effective CAPA.
Have you encountered this mindset?
If you are facing a troubling result or need to ensure your OOS and deviation investigation systems are audit-ready, reach out to us today!